Our research is driven by our interest for the coupling between the small scale physical, biological and chemical mechanisms underlying the processes that control soil-like systems, characterised by confined fluids flow, transport & mixing. We use and develop laboratory experiments (mostly microfluidics and video-microscopy), numerical simulations and minimal-ingredient theoretical models. The comparison between micro-scale and field data represents a first step in discovering the laws linking the micro-scale processes to the larger scale behavior, which is not direct in general. The upscaling issue is a major component of our research.
=======
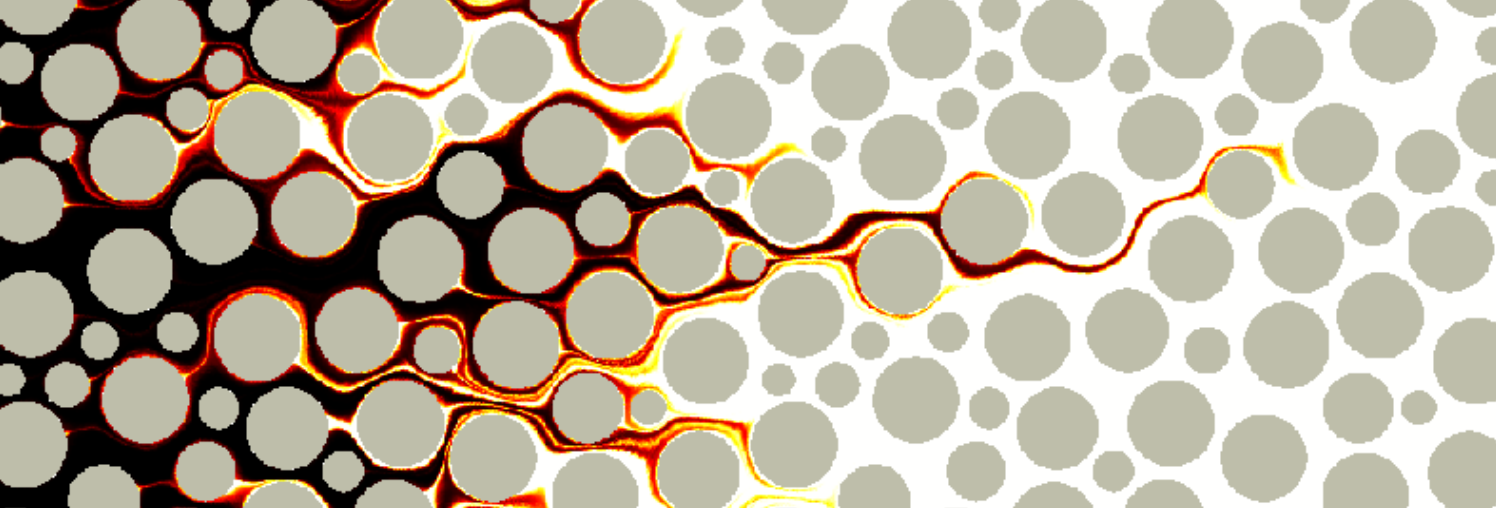
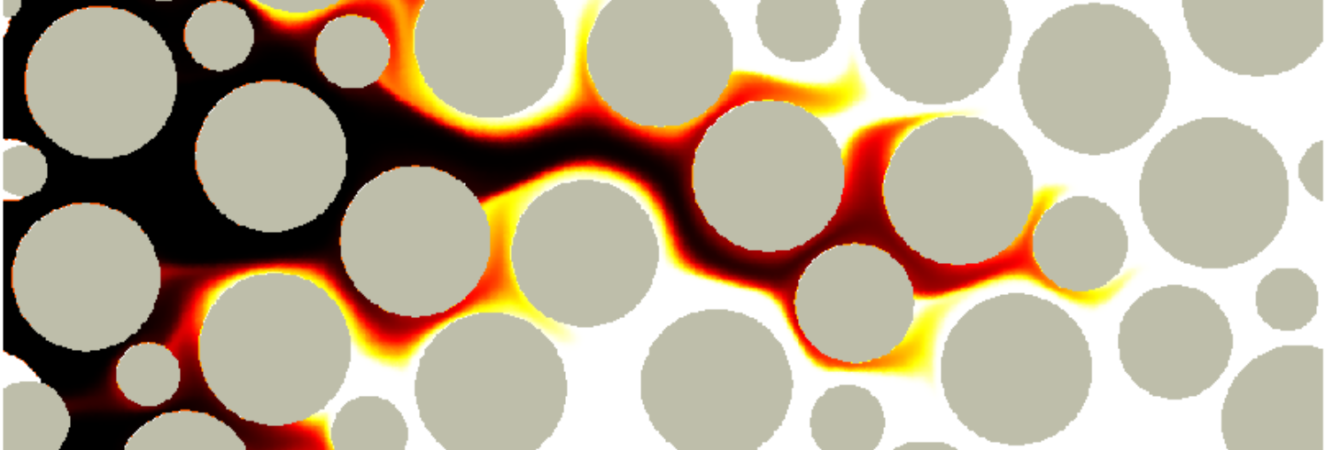
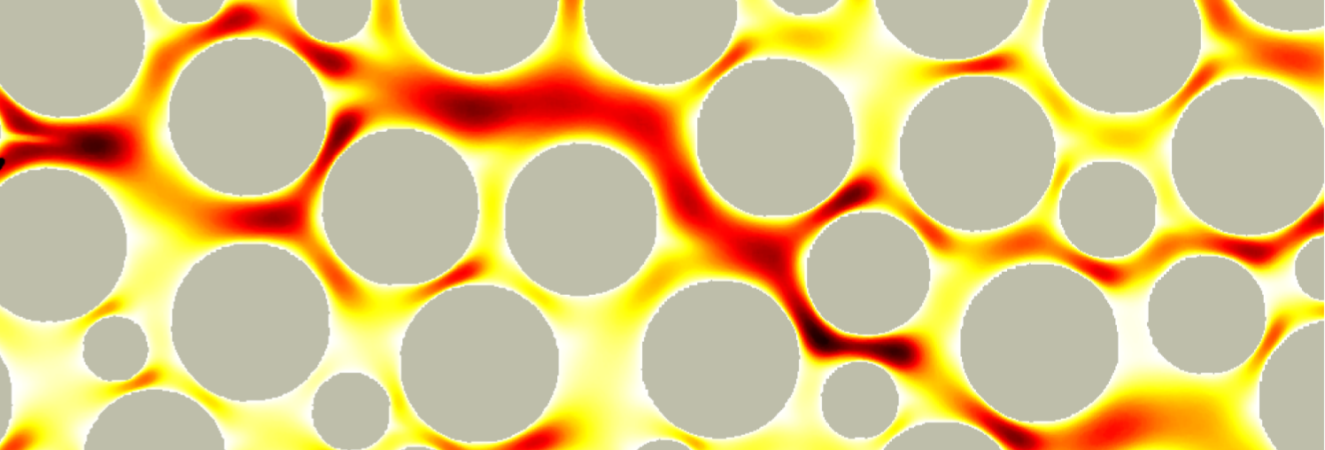
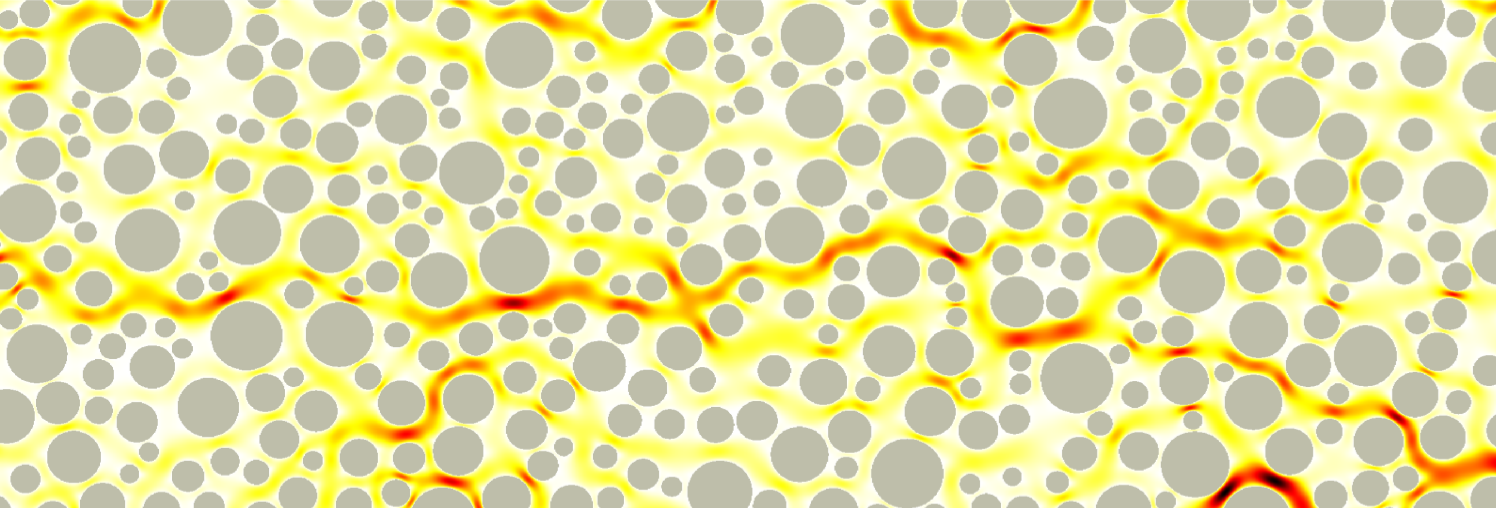
Vortices in dead-end pores control anomalous dispersal
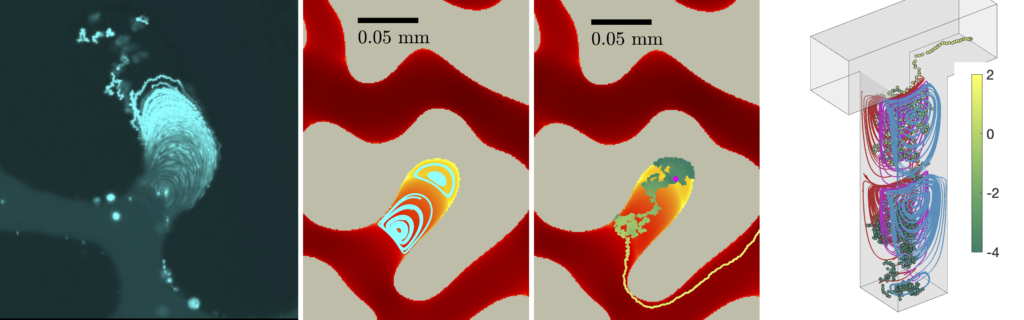
The role of microscopic structure and flow for the dispersal of particles and solutes in disordered systems characterized by dead-end pores has been only poorly understood, in part due to the stagnant nature of these microscopic regions in the global scale of the system. We study how particles are transported, retained and dispersed in such systems. We observe strong tailing of arrival time distributions at the outlet of the medium characterized by power-law decay with an exponent of 2/3. Our results demonstrate how microscopic flow structures can impact macroscopic particle transport. This work in under review.
Microbial transport & chemotaxis
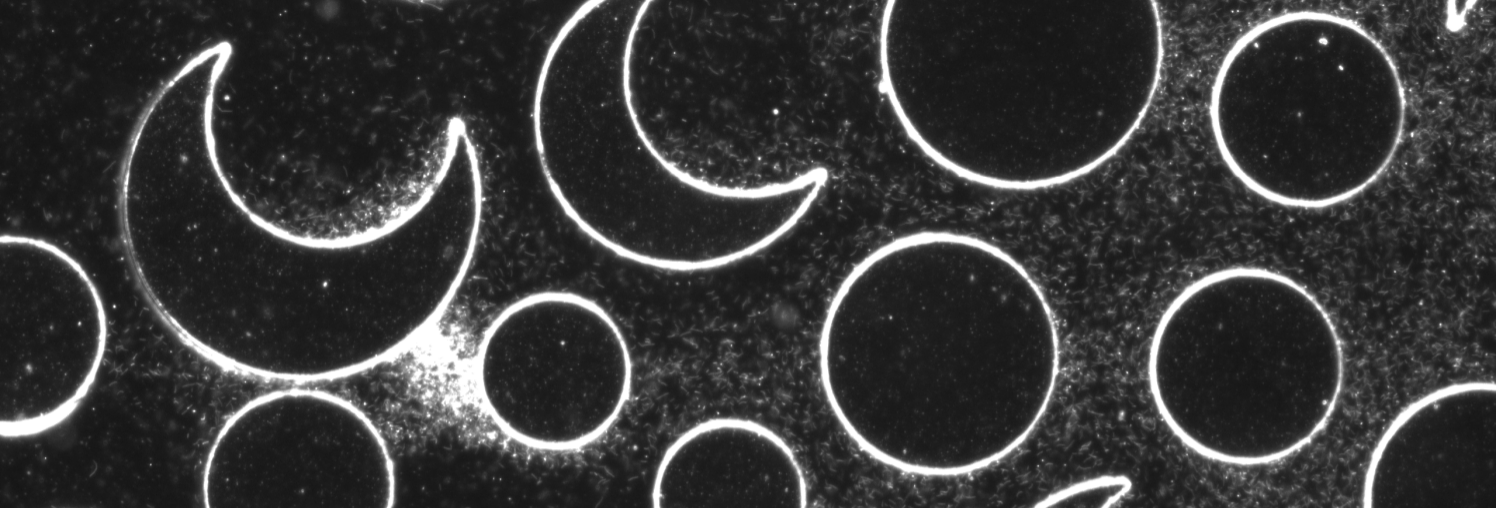
Natural soils are host to a high density and diversity of microorganisms, and even deep-earth porous rocks provide a habitat for active microbial communities. In these environments, microbial transport by disordered flows is relevant for a broad range of natural and engineered processes, from biochemical cycling to remineralization and bioremediation. Yet, how bacteria are transported and distributed in the sub- surface as a result of the disordered flow and the associated chemical gradients characteristic of porous media has remained poorly understood, in part because studies have so far focused on steady, macroscale chemical gradients. Using a microfluidic model system that captures flow disorder and chemical gradients at the pore scale we quantify the transport and dispersion of the soil-dwelling bacterium Bacillus subtilis in porous media. Read more here.
Bacterial dispersal in porous media
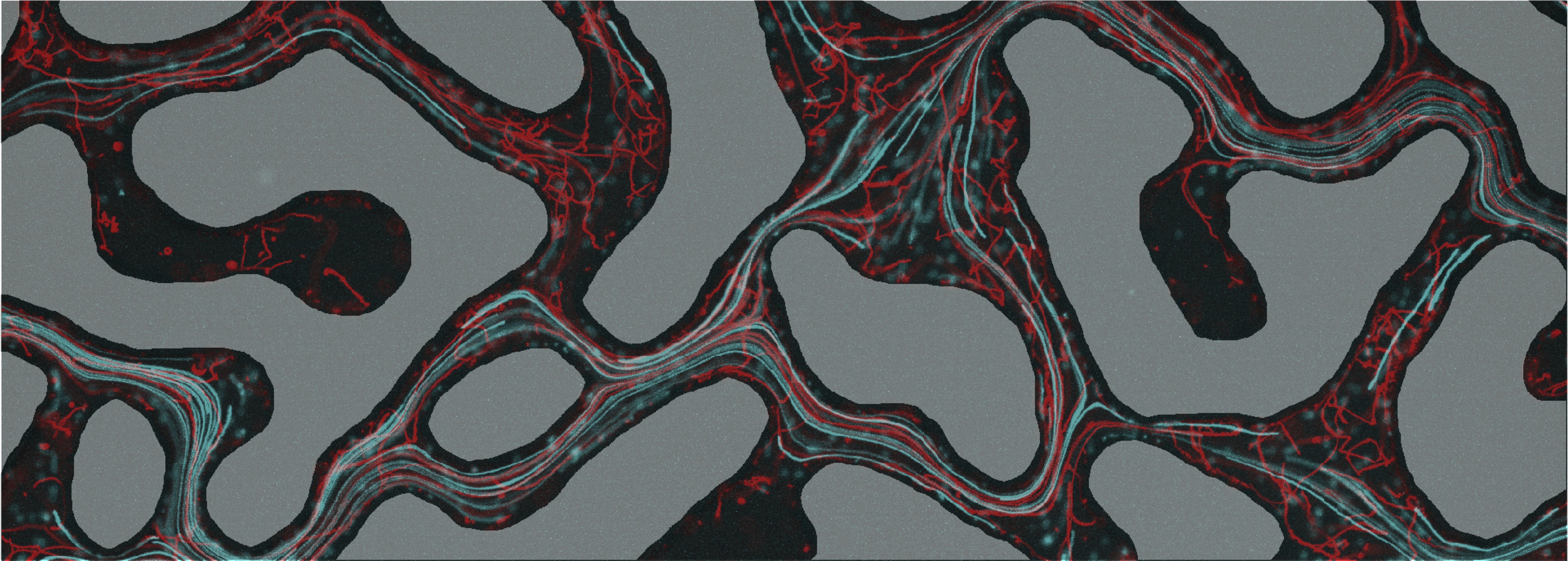
Biological tissues, sedimentary environments, or engineered systems may present topological features that promote the coexistence of transmitting pores connecting dead-end pores, those that cannot host a net fluid flow. This complex physical environment may have repercussion on the hosted microorganisms by controlling the transport of individuals (i.e. dispersal), resources availability, as well as chemical signals produced by bacteria. The image above shows tracks of motile bacteria (in red) and non-motile ones (in blue) while transported by the fluid flow. See more here.
Microbial attachment & Biofilm formation
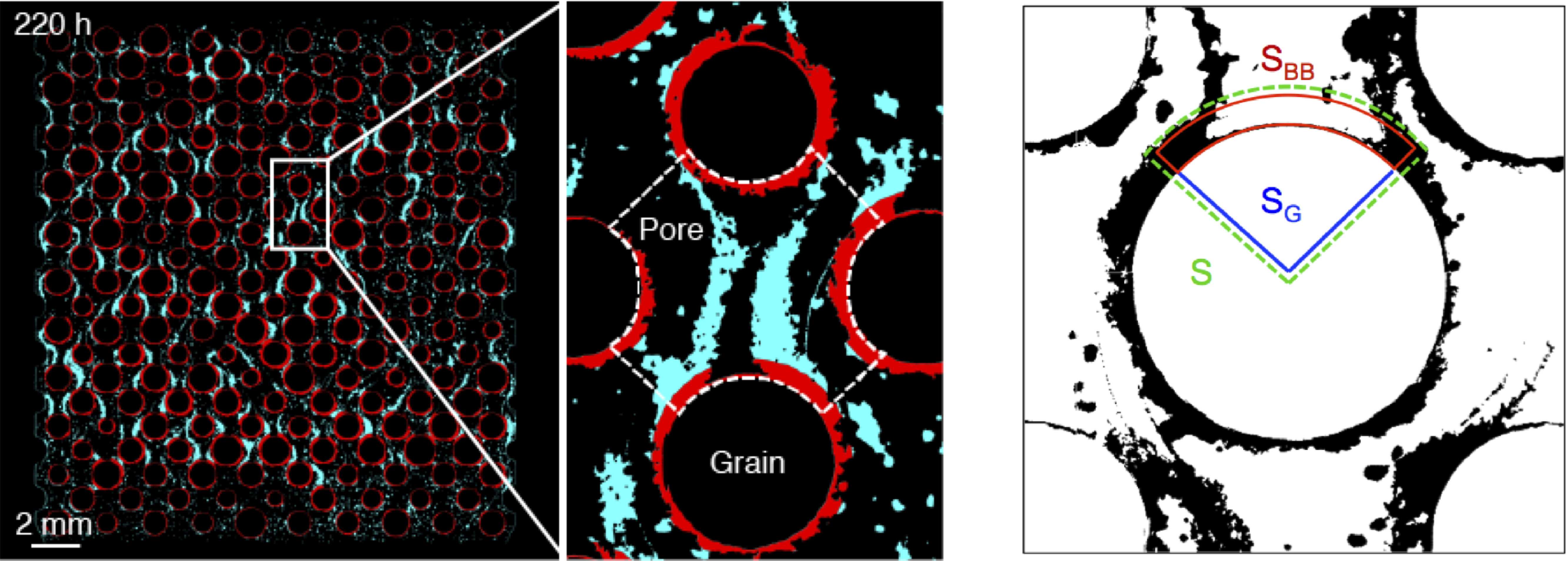
The evolution of biofilm structures within rough fracture or porous media flows is different from the one observed in absence of fluid flow on a flat surface. The heterogeneous flow conditions, the transport of nutrients and the mechanical fluid stresses are coupled to the formation and the evolution of biofilms that can also filter transported bacteria. For instance, biofilm structures may partially detach from the solid walls to occupy the pore space with importance consequences on fluid flows, nutrient transport and medium clogging. See more here.
Mixing in confined flows
Despite the low Reynolds number characterising porous media flows, transport and mixing of solutes and microbes suspensions in confined environments is non trivial. While some mixing properties are shared with turbulent and chaotic systems, there are fundamental differences that must be taken into account to properly model and predict the dynamics of mixing in such media, including the presence of solid boundaries, no flow zones and different flow kinetics and distributions. See more about experimental results here and theoretical models here. We recently published about diffusion-limited mixing in confined spaces here.
Confined flow complexity
Flow structures in confined media lead to localised regions and temporal windows for which the transport is qualitatively and quantitatively different from the average. The complexity of confined flow topologies is fundamentally different from the one resulting in open chaotic or turbulent ones and arise from the random spatial organisation of the host medium and the interaction between the mobile (fluid) and non mobile (solid or trapped fluid) part of the system. See more here.
Horizontal Gene Transfer in confined micro-structures

Soil microbial communities are exposed to transported portions of DNA, Mobile Genetic Elements (MGE), which in case of environmental stress can be incorporated by the microbes with a consequent appearance of new physiological functions. The mechanisms responsible for such (horizontal) gene transfer are controlled by the environmental conditions. Thus, due to the complexity of small scale confined flow, the predictions based on rates, measured in well-mixed batch reactors, may differ by orders of magnitudes from observations in the field.
Microfluidics
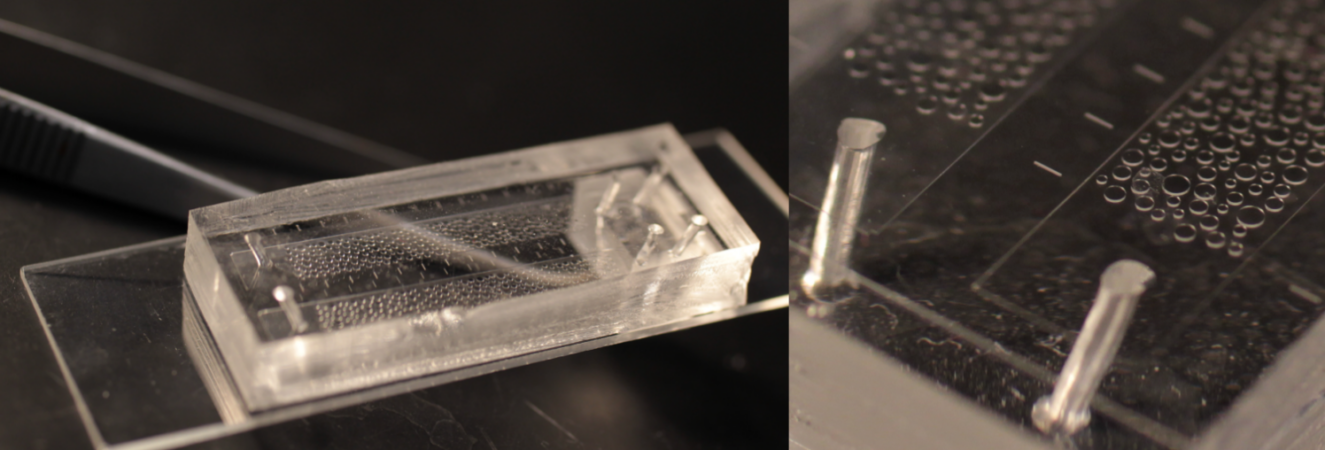
Boreholes drilled into the ground represent traditionally the main source of information about subsurface processes and phenomena, due to the opaque nature of soil and rocks. We build two dimensional micro-models analogs of simplified confined media that consist in two parallel (at least one transparent) solid layers separated by a thin gap filled with impermeable solid obstacles, whose size and shape are designed at will using photo-lithography in a range between micrometers to millimetres.